
CUPISCO Study
THE CUPISCO STUDY
The study title
The phase II CUPISCO study (NCT03498521) compares the efficacy and safety of targeted therapy or immunotherapy guided by CGP vs platinum-based standard chemotherapy in patients with poor prognosis CUP who have received 3 cycles of platinum doublet induction therapy.

Clinicaltrials.gov identifier: NCT03498521. Available at clinicaltrials.gov/ct2/show/NCT03498521.
BACKGROUND INFORMATION
Approximately 85% of CUP cases are assumed to have a clinically relevant genomic alteration and may respond to molecularly-guided therapy.1 The phase II CUPISCO study is evaluating the efficacy and safety of molecularly-guided therapy after CGP in CUP patients with poor prognosis.2
-
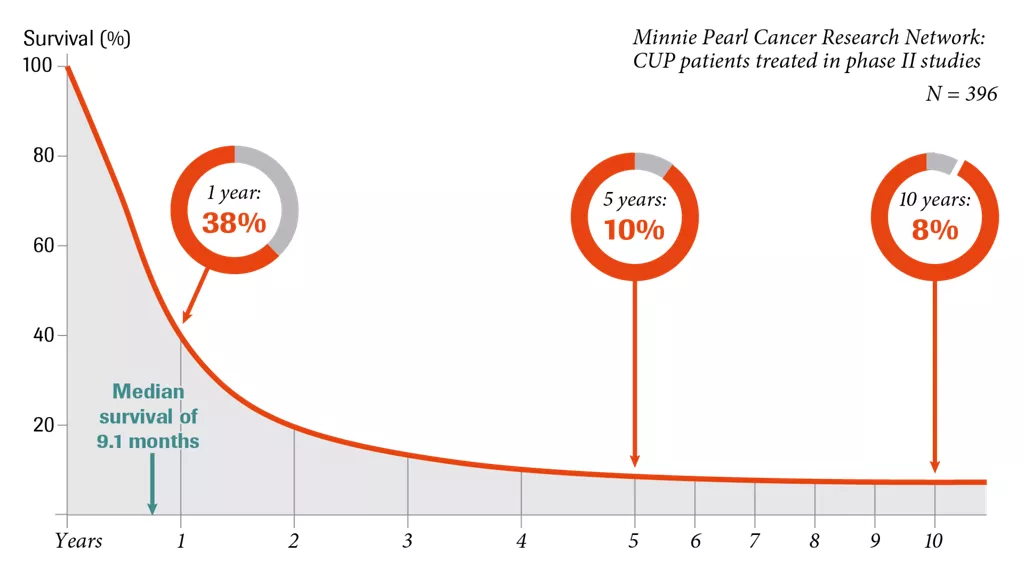
What are the survival rates for CUP patients with poor prognosis?
The survival rates for patients with poor prognosis are low:3
- 1 year: 38%
- 5 years: 10%
- 10 years: 8%
-
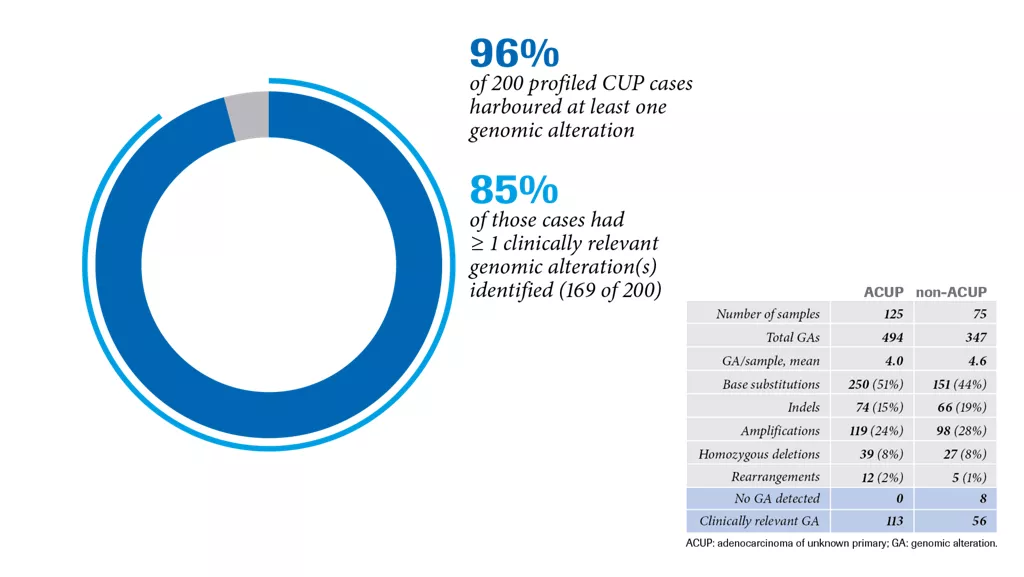
What is the potential of identifying genomic alterations in CUP?
Most CUP samples exhibit genomic alterations. For example, as described in a publication by Ross JS et al. samples from 200 CUP cases were analysed with CGP (Foundation Medicine tissue biopsy assay) to determine whether it is possible to identify genomic alterations that can serve as a basis for targeted therapy.1 Of the 200 CUP cases, 96% exhibited at least one genomic alteration, with one or more clinically relevant genomic alterations identified in 85% of these cases (169 of 200).
-
WHAT ARE THE MUTUAL BENEFITS FOR CGP AND CUP?
CUP may be a potential model disease for clinical utility of CGP in a pan-tumour fashion, independent from the site of tumour origin. Simultaneously, CGP can direct new treatment options in patients with CUP.
1. Ross JS et al. JAMA Oncol 2015; 1: 40–9.
2. Clinicaltrials.gov identifier: NCT03498521. Available at: clinicaltrials.gov/ct2/show/NCT03498521.
3. Greco FA and Hainsworth JD (2011) Cancer of unknown primary site, DeVita VT Jr., Hellman S, Rosenberg SA (eds) Cancer: Principles and Practice of Oncology (9th ed) Philadelphia, PA, JB Lippincott: 2033–51.
RATIONALE FOR A STUDY IN CUP PATIENTS
What is the rationale for the CUPISCO study?
CGP could offer patients with poor prognosis CUP new options for treatment since it permits the identification of potentially clinically relevant alterations in CUP.1-6 The main objective of the study is to determine the efficacy and safety of molecularly-guided therapies based on CGP vs a standard chemotherapy regimen.7

1. Frampton GM et al. Nat biotechnol 2013; 31: 1023–31.
2. He J et al. Blood 2016; 127: 3004–14.
3. Gagan J and van Allen EM. Genome Med 2015; 7: 80.
4. Rozenblum AB et al. J Thorac Oncol 2017; 12: 258–68.
5. Suh JH et al. Oncologist 2016; 21: 684–91.
6. Ettinger DS et al. NCCN Guidelines version 2.2019.
7. Clinicaltrials.gov identifier: NCT03498521. Available at clinicaltrials.gov/ct2/show/NCT03498521.
8. Ross JS et al. JAMA Oncol 2015; 1: 40–9.
9. Kato S et al. Cancer Res 2017; 77: 4238–46.
10. Krämer A et al. J Clin Oncol 2018; 36: 15 (suppl e24162).
CUPISCO STUDY DESIGN
The CUPISCO study will recruit a total of 790 patients. The planned study duration will be approximately 48 months.1
Tumour profiles of CUP patients are determined from tissue and blood samples using the Foundation Medicine tissue biopsy assay and Foundation Medicine liquid biopsy assay, respectively.2
After 3 cycles of platinum doublet (carboplatin/paclitaxel, cisplatin/gemcitabine or carboplatin/gemcitabine) induction therapy, the responder patients are randomised:1
- Experimental arm: targeted therapy tailored to the molecular profile
- Standard arm: continuation of systemic chemotherapy
The non-responder patients go directly to molecularly-guided therapy (according to their genomic profile).

What is the CUPISCO study design and what are the study endpoints?
The study endpoints are:
- Primary endpoint: progression-free survival (PFS)
- Secondary endpoints:
- Overall survival (OS)
- Overall response rate (ORR)
- Duration of clinical benefit (DCB)
- Adverse events (AEs)1
1. Clinicaltrials.gov identifier: NCT03498521. Available at: clinicaltrials.gov/ct2/show/NCT03498521.
2. Foundation Medicine, Inc. 2018. Available at: www.foundationmedicine.com (last accessed March 2019).
KEY CUPISCO STUDY CRITERIA
On the right-hand side you can see a selection of key inclusion criteria for the CUPISCO study.

On the right-hand side you can see a selection of key exclusion criteria for the CUPISCO study.

Clinicaltrials.gov identifier: NCT03498521. Available at: clinicaltrials.gov/ct2/show/NCT03498521.
CENTRAL ELIGIBILITY REVIEW PROCESS
How is trial eligibility decided?
The eligibility review process allows for patient enrolment in the CUPISCO study according to ESMO clinical guidelines.1

1. Krämer A et al., Annals of Oncology 2022/ https://www.annalsofoncology.org/article/S0923-7534(22)04769-X/fulltext
FOUNDATION MEDICINE'S CURATED CGP REPORT
What is provided in the CGP report from Foundation Medicine?
CGP results will be provided in a clear, in-depth report that offers information, including tumour mutational burden, microsatellite instability and genomic alterations. Treatment options based on approved therapies or clinical trials are also provided.

Sample report as used in CUPISCO study.
THE MOLECULAR TUMOUR BOARD
What is the role of the molecular tumour board (MTB)?
The MTB consists of medical experts in CUP oncology and pathology. It aims to provide independent guidance to the investigator regarding the choice of molecularly-guided therapy based on the CGP report.

Roche data on file, Molecular Tumor Board Charter MX39795 v9.
CUPISCO STUDY SITES
What is the reach of the CUPISCO study?
CUPISCO is a global study with 153 sites planned to participate in 34 countries.

